Research in our lab is focusing on the mechanisms of Chromosomal Instability (CIN), which is a hallmark of human cancer and a key driving force for tumorigenesis, tumor progression and tumor evolution. CIN causes the perpetual generation and further evolvement of structural and numerical chromosome aberrations that directly contribute to phenotypic changes in tumor cells towards the development of aggressive and therapy-resistant forms of cancer. Thus, it is of utmost interest to understand how CIN is triggered and how CIN might be targeted through novel therapeutic concepts.
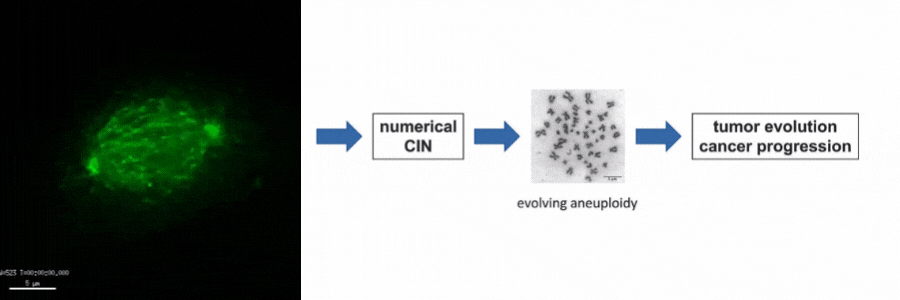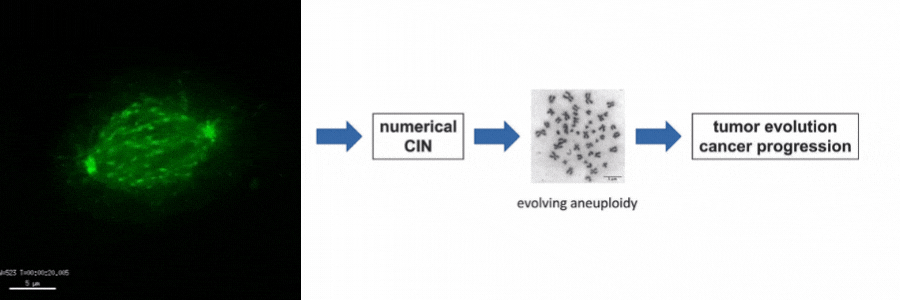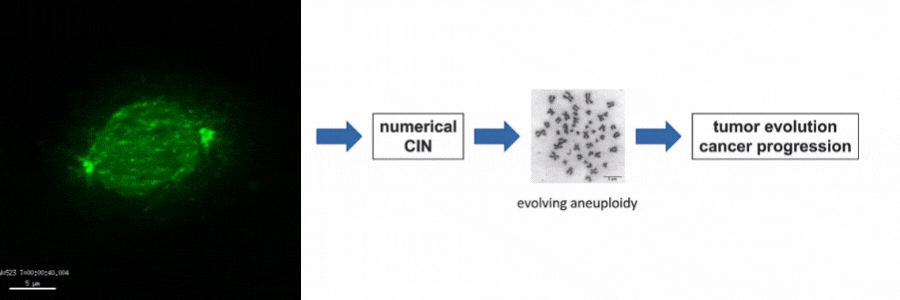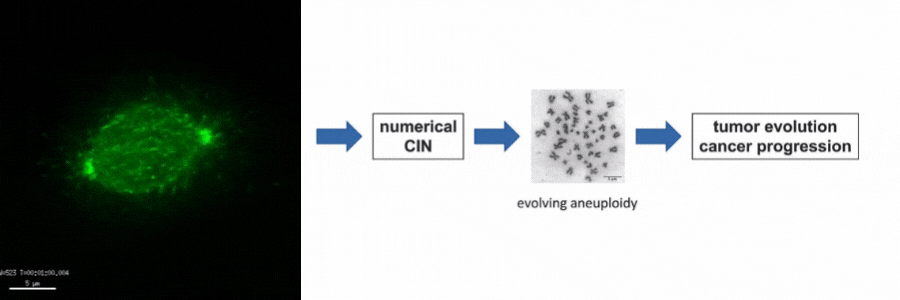
Our lab is particularly interested in the mechanisms of how numerical CIN, which is caused by errors during mitotic chromosome segregation, is generated in human cancer cells. For this, we are investigating the role of dynamic microtubules in mitosis, the assembly of the mitotic spindle, the links between DNA replication stress and mitotic dysfunction and the role of oncogenic and tumor suppressor pathways in mitotic regulation. Together, we aim to understand of how structural and numerical chromosome aberrations as driving forces for tumor evolution are generated in human cancer cells.

contact information
- telephone: +49 551 3923823
- e-mail address: holger.bastians(at)uni-goettingen.de
Address
Grisebachstraße 8
37077 Göttingen
Principle Investigator
Holger Bastians is a Professor for Cellular Oncology at the University Medical Center Göttingen (UMG) and at the Göttingen Center for Molecular Biosciences (GZMB).
He studied Biology with a focus on molecular biology and genetics at the Universities in Bayreuth and Osnabrück.
He obtained his Ph.D. (summa cum laude) at the German Cancer Research Center (DKFZ) in Heidelberg in 1996, where is investigated the role of novel protein phosphatases in human cell cycle control.
In 1996, he moved to Boston, USA, to join the laboratory of Prof. Joan Ruderman at the Harvard Medical Center as a postdoctoral fellow. His work was focusing on the role and mechanisms of mitosis-specific protein proteolysis mediated by the anaphase promoting complex or cyclosome (APC/C) in human cells.
Following his postdoctoral work in the US, he became an independent group leader at the Institute of Molecular Tumor Research (IMT) at the Philipps-University in Marburg. With his own lab, he investigated the role of the mitotic spindle checkpoint and the mechanisms of chromosome missegregation in human cancer cells.
In 208, Holger Bastians was awarded with the prestigious DFG Heisenberg fellowship and in 2011, he became a Heisenberg Professor at the University Medical Center Göttingen. Since 2013 he is an appointed Professor for Cellular Oncology at the UMG and GZMB.
Based on his research work, Holger Bastians was awarded in 2015 with the Innovation Prize of the German Society for Cell Biology (DGZ).
Since 2019 he is the speaker of the DFG-funded research unit 2800 (FOR2800) “Chromosome Instability: cross-talk of DNA replication stress and mitotic dysfunction”. Additionally, he is heading research projects as part of the DFG-funded Clinical Research Unit 5002 (KFO5002, since 2020) and within the Collaborative Research Center 1324 (SFB1324, since 2017).
Publications
- Böhly, N.*, Schmidt, A.K.*, Zhang, X.*, Slusarenko, B.O., Hennecke, M., Kschischo, M. and H.Bastians. Increased replication origin firing links replication stress to whole chromosomal instability in human cancer. Cell Reports 41: 111836.
- Schmidt, A.K., Pudelko, K., Boekenkamp, J.E., Berger, K., Kschischo, M. and H.Bastians. 2021. The p53/p73 - p21CIP1 tumor suppressor axis guards against chromosomal instability by restraining CDK1 in human cancer cells. Oncogene 40: 436-451.
- Lin, Y.C., Haas, A., Bufe, A., Parbin, S., Hennecke, M., Voloshanenko, O., Gross, J., Boutros, M., Acebron, S.P. and H.Bastians. 2021. Wnt10b-GSK3β-dependent Wnt/STOP signaling prevents aneuploidy in human somatic cells. Life Sci Alliance 4: e202000855.
- Böhly, N., Kistner (Hennecke), M. and H.Bastians. 2019. Mild replication stress causes aneuploidy by deregulating microtubule dynamics in mitosis. Cell Cycle 18: 2770-2783.
- Ertych, N., Stolz, A., Valerius, O., Braus, G.H. and H. Bastians. 2016. The CHKK2-BRCA1 tumor suppressor axis restrains oncogenic AURORA-A to ensure proper mitotic microtubule assembly. Proc. Nat. Acad. Sci. USA. 113:1817-1822.
- Lüddecke, S., Ertych, N., Stenzinger, A., Weichert, W., Beissbarth, T., Dyczkowski, J., Gaedcke, J., Valerius, O., Braus, G.H., Kschischo, M. and H. Bastians. 2016. The putative oncogene CEP72 inhibits the mitotic function of BRCA1 and induces chromosomal instability. Oncogene. 35:2398-2406.
- Stolz, A., Neufeld, K., Ertych, N. and H. Bastians. 2015. Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep. 16:490-499.
- Ertych, N., Stolz, A., Stenzinger, A., Weichert, W., Kaulfuß, S., Aigner, A., Wordeman, L. and H. Bastians. 2014. Increased microtubule assembly rates influence chromosomal instability in colorectal cancer. Nature Cell Biology. 16:779-791.
- Stolz, A., Ertych, N., Kienitz, A., Vogel, C., Schneider, V., Fritz, B., Jacob, R., Dittmar, G., Weichert, W., Petersen, I. and H. Bastians. 2010. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nature Cell Biology. 12:492-499.
For full publication list, please visit pubmed
Contact
Mailing address:
University Medical Center Göttingen
Institute of Molecular Oncology
Section for Cellular Oncology
Grisebachstraße 3,
37077 Göttingen, Germany
We are located on the 3rd floor of the building “Institute of Microbiology and Genetics” on the North Campus of the Georg-August University (Grisebachstrasse 8)
For general lab enquiries please contact:
Magdalena Hennecke, lab technician
Email: magdalena-isabell.hennecke(at)zentr.uni-goettingen.de
Group members

contact information
- telephone: +49 551 3929654
- e-mail address: randolf.bodenstein(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: simranjeet.kaur(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: atmika.paul(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: gargee.joshi(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: francesca.razzano(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: friederike.wenz(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: lennart.wrachtrup(at)med.uni-goettingen.de

contact information
- telephone: +49 551 3929654
- e-mail address: shengyu.yao(at)med.uni-goettingen.de
Funding
Work in the Bastians lab is supported by the Deutsche Forschungsgemeinschaft:
1. DFG-funded Research Unit 2800 (Forschungsgruppe 2800, FOR2800)
„Chromosome Instability: Cross-talk of DNA replication stress and mitotic dysfunction”.
Speaker: Holger Bastians
Our lab established and coordinates this nation-wide research consortium. In our FOR2800 sub-project (SP2) we are focusing on the molecular mechanisms of how DNA replication stress causes mitotic chromosome missegregation leading to the induction of chromosomal instability in human cancer cells.
For further information please visit: https://for2800.de/

2. DFG-funded Clinical Research Unit 5002 (Klinische Forschungsgruppe 5002, KFO5002)
„Characterization and targeting of genome dynamics for sub-type specific therapy of pancreatic cancer”.
In our KFO5002 sub-project (B03) we are focussing on the role of tumor suppressor signaling in genome instability in pancreatic cancer.
For further information please visit https://gccc.umg.eu/kfo5002

3. DFG-funded Collaborative Research center 1324 (Sonderforschungsbereich 1324, SFB1324)
„Mechanisms and functions of Wnt signaling”.
In our SFB1324 sub-project (B03) we are focussing on the mitotic role of Wnt signaling and elucidate how Wnt signaling maintain genome stability.
For further information please visit: https://sfb1324.de/

4. DFG-funded project “Analysis of the genome-stabilizing function of the tumor suppressor BRCA1”.
In our DFG-project we are investigating how BRCA1 acts as genome stabilizer and how it regulates mitotic chromosome segregation in order to ensure chromosome stability.

5. NSF-DFG-funded collaboration project “The role of microtubule-actin cross-talk in the regulation of mitosis” (together with Prof. Linda Wordeman, Seattle, USA)
In our joint project funded by the National Science Foundation (NSF, USA) and the DFG we are collaborating with Prof. Linda Wordeman (Seattle, WA. USA) to investigate how spindle orientation is regulated in mitosis by microtubule and actin networks to ensure proper chromosome segregation and genome stability.




